Biomarker Predicts Risk of Preterm Birth Earlier
By LabMedica International staff writers
Posted on 26 Jan 2016
A standard biomarker test offered earlier in pregnancy could potentially help doctors to better identify women at risk of giving birth prematurely, thus enabling health services to focus treatments on women at highest risk.Posted on 26 Jan 2016
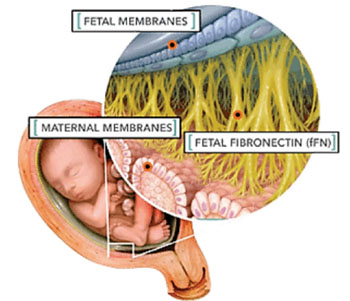
A number of factors are used to determine if a woman is at risk of giving birth prematurely, including a history of preterm births or late miscarriages. Two further factors which clinicians normally consider are the length of cervix and levels of a biomarker found in vaginal fluid known as fetal fibronectin.
Scientists at King's College London (London, UK) compared measurements of a new fetal fibronectin test in the cervicovaginal fluid of women at 18 to 21 weeks of gestation with measurements made at 22 to 27 weeks of gestation, to see which time period offered the best prediction of spontaneous preterm birth. They also explored whether using a low (10 ng/mL) and high (200 ng/mL) threshold would more accurately classify a women's risk of giving birth prematurely.
Fetal fibronectin was measured using Hologic 10Q system (Hologic; Marlborough, MA, USA). Of the 898 high risk women followed in the study, 8.7% delivered spontaneously before 34 weeks of gestation and only 3.8% of women with concentrations less than 10 ng/mL (tested at 18–21 weeks) delivered before 34 weeks of gestation, a similar rate to that expected in a normal pregnancy. This compared to 2.9% in those tested later (22–27 weeks). In those woman over 200 ng/mL, similar proportions delivered after 34 weeks whether tested early or late (39% versus 43%).
The authors concluded that measuring fetal fibronectin at 18–21 weeks pregnancy appears to offer a similar predictive value to measurements at 22–27 weeks. For both the early and standard tests, there was a noticeable increase in relative risk between the lowest and highest thresholds used in the study. Combining cervical length with the biomarker test was found to improve the diagnostic accuracy further. Low fibronectin concentrations are associated with spontaneous preterm birthrates approaching population background levels.
Andrew A. Shennan, MD, a professor of Obstetrics and lead author of the study, said, “The aim of our study was to find better ways of establishing the risk of women giving birth prematurely in early pregnancy, to enable us to focus on the women most likely to benefit from earlier intervention and close monitoring during pregnancy. Instead of relying on the traditional single threshold later in pregnancy we now can more accurately identify those likely to be normal and those most in need of early interventions, from the first half of pregnancy.” The study was published on January 7, 2016, in the journal Obstetrics and Gynecology.
Related Links:
King's College London
Hologic