Performance Evaluated for Point-of-Care HbA1c Analyzer
By LabMedica International staff writers
Posted on 08 Jul 2015
Glycated hemoglobin (HbA1c) levels, which reflect the average plasma glucose concentration over the preceding three months, play a pivotal role in the diagnosis, assessment, and monitoring of diabetes.Posted on 08 Jul 2015
The immediate feedback of HbA1c levels is highly effective for controlling plasma glucose levels and point-of-care (POC) instruments, characterized as fast, portable, and easy-to-use, have been shown to be suitable for providing rapid feedback of HbA1c levels.
Medical laboratory scientists at the Seoul National University College of Medicine (Republic of Korea) assessed the analytical performance of a newly developed POC HbA1c analyzer. To investigate the accuracy of the analyzer, they measured the deviation from the International Federation of Clinical Chemistry (IFCC) reference targets using two sets of 12 duplicate fresh venous whole blood specimens with a range of 33.3 mmol/mol to 91.3 mmol/mol in International System of Units (SI) units, or 5.2% to 10.5% in National Glycohemoglobin Standardization Program (NSGP) units.
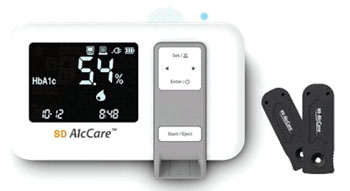
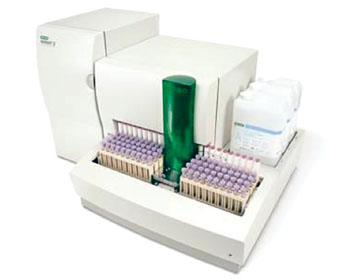
The SD A1cCare analyzer (SD Biosensor, Inc.; Suwon, Korea), which utilizes an immunochromatography assay, was used to measures total hemoglobin and HbA1c levels via its optical system and calculates the percentage HbA1c. The SD A1cCare Test kit uses an anti-HbA1c antibody that is specific for the first few amino acid residues of the glycated N-terminus of the β-chain of hemoglobin A0. The scientists tested 150 residual specimens using both the SD A1cCare and the Variant II Turbo (Bio-Rad Laboratories, Inc.; Hercules, CA, USA) in 14 days.
The SD A1cCare POC HbA1c analyzer showed excellent precision, linearity and correlation with Variant II Turbo high-performance liquid chromatography (HPLC) and it showed excellent accuracy with IFCC targets. Deviations from IFCC targets at 30, 60, and 90 mmol/mol IFCC levels were − 1.95, − 1.85, and − 1.74 mmol/mol, respectively. The coefficients of variation based on EP9-A2 protocol were 2.6% in SI units and 1.8% in NGSP units.
The authors concluded that the SD A1cCare analyzer showed excellent precision, linearity, correlation with the Variant II Turbo analyzer, and accuracy with IFCC targets and therefore, it may be suitable for HbA1c assays in the POC setting and in small laboratories. The study was published in the June, 2015 issue of the journal Clinical Biochemistry.
Related Links:
Seoul National University College of Medicine
SD Biosensor Inc.
Bio-Rad Laboratories