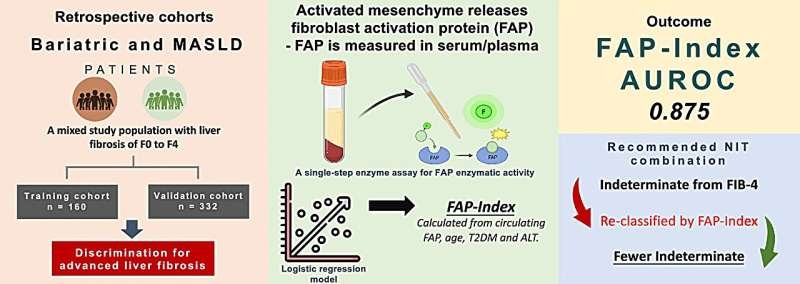
Dedicated Blood DNA Collection Tube Receives European Marketing Approval
By LabMedica International staff writers
Posted on 13 Feb 2014
A new collection tube for routine clinical use that was designed to preserve DNA in blood samples was recently certified for use as a diagnostic product in Europe.Posted on 13 Feb 2014
The PreAnalytiX GmbH (Feldbachstrasse, Switzerland) PAXgene Blood DNA Tube provides a means for the collection of whole blood for the isolation of genomic DNA in a closed, evacuated system. Blood is collected under a standard phlebotomy protocol into an evacuated tube that contains potassium-EDTA additive. Complete isolation of DNA is carried out using manual or automated methods such as salting out precipitation, magnetic beads, or silica membrane-based technologies.
Image: The PAXgene Blood DNA Tube (Photo courtesy of PreAnalytiX GmbH).
The 2.5 milliliter PAXgene Blood DNA Tube stabilizes genomic DNA with validated time and temperature parameters. The tube incorporates workflow efficiency features including a two-dimensional bar code on the label and a tube closure that clearly identifies this unique DNA collection tube.
The PAXgene tube, which recently received the European Union's CE marking IVD certification for sale in Europe was developed as part of a joint venture between Becton Dickinson and Company ((Franklin Lakes, NJ, USA) and QIAGEN (Venlo, The Netherlands).
“The validated performance data we provide to clinical scientists for the PAXgene Blood DNA Tube should reduce the chances of preanalytical error, ensuring the best quality sample for accurate IVD test performance and for blood DNA storage related to the identification of new biomarkers of disease or drug actions,” said Frank Augello, general manager of PreAnalytiX GmbH.
Related Links:
PreAnalytiX GmbH
Becton Dickinson and Company
QIAGEN