Novel Biomarker Predicts Steatohepatitis Severity
By LabMedica International staff writers
Posted on 21 Nov 2013
Liver biopsy remains the gold standard for the nonalcoholic steatohepatitis (NASH) diagnosis and the grading liver disease severity in nonalcoholic fatty liver disease (NAFLD) patients.Posted on 21 Nov 2013
However, invasive liver biopsy is poorly suited as a diagnostic test for such a prevalent condition, and this, in turn, restricts therapeutic intervention. The development and validation of a reproducible and noninvasive test that can accurately distinguish NASH from simple steatosis is urgently needed.
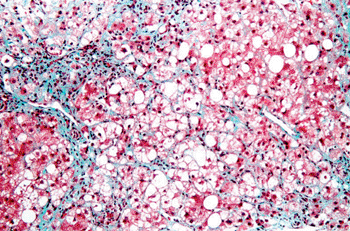
Image: Micrograph of a liver biopsy showing steatohepatitis (Photo courtesy of Nephron).
Scientists at Osaka University (Japan) enrolled 127 biopsy-proven NAFLD patients and 23 normal controls (NC) in a study to search for a suitable biomarker. For all patients, biochemical variables were measured using a conventional automated analyzer. The tests included were for platelet counts, aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transpeptidase, albumin, total cholesterol, triglyceride, fasting blood glucose, immunoreactive insulin, ferritin, and hyaluronic acid.
The scientists developed their own enzyme-linked immunoassay (ELISA) to measure the levels human macrophage-associated lectin binding protein (Mac-2bp) in the blood of the enrollees. Mac-2bp is one of the major fucosylated glycoproteins, which were identified with glycol-proteomic analyses. The ELISA assay system was finally designed as a commercial kit and galectin-3 was also measured using Human Galectin-3 Assay kit from the same company (Immuno-Biological Laboratory; Fujioka, Japan). For the quantitative measurement of the caspase-generated neoepitope of cytokeratin 18 (CK-18), they used the M30-Apoptosense ELISA (Peviva AB; Bromma, Sweden).
Serum Mac-2bp levels were significantly increased in non-NASH patients to 1.103 ± 0.5 μg/mL compared with normal control at 0.675 ± 0.27 μg/mL. The serum Mac-2bp levels in NASH patients exhibited greater increases to 2.132 ± 1.24 μg/mL than did those in non-NASH patients. Serum M30 antigen levels were also significantly higher in NASH patients than in non-NASH patients. Multivariate logistic regression analyses showed Mac-2bp levels could predict the fibrosis stage and the presence of ballooning hepatocytes in NAFLD patients.
The authors concludes that despite some limitations, serum Mac-2bp levels could distinguish NASH from non-NASH patients and estimate the disease severity of NAFLD patients with accuracy superior to that of the M30 antigen in their patients. Further investigation is needed using a larger number and wider spectrum of NAFLD patients. The study was published online on September 17, 2013, in the journal Proteomics Clinical Applications.
Related Links:
Osaka University
Immuno-Biological Laboratory
Peviva AB










 Analyzer.jpg)



