Phosphorylation of Tau Protein Inhibits Amyloid-beta Toxicity in Alzheimer’s Model
By Gerald M. Slutzky, PhD
Posted on 29 Nov 2016
A team of Australian Alzheimer's disease (AD) researchers have presented evidence suggesting that phosphorylation of tau protein in the early stages of the disease acts to protect against the toxicity of amyloid-beta (Abeta) plaques, and that this protective effect disappears as the disease progresses.Posted on 29 Nov 2016
The prevailing idea among AD researchers has been that Abeta induced phosphorylation of tau, which in turn triggered the neuronal dysfunction that characterized the disease.
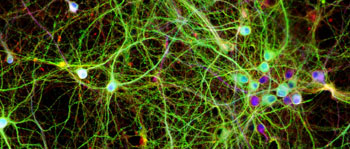
Image: A photomicrograph of neurons growing in culture. The colors highlight the human tau protein in green, a structural component in red, and the DNA inside the cell nucleus in blue (Photo courtesy of Dr. Lars Ittner, University of New South Wales).
Investigators at the University of New South Wales (Sydney, Australia) worked with Alzheimer's disease mouse models and samples of brain tissue obtained from AD patients. They reported in the November 18, 2016, issue of the journal Science that at least in early stages of the disease, site-specific phosphorylation of tau inhibited Abeta toxicity. This specific tau phosphorylation was mediated by the neuronal enzyme p38gamma (p38 mitogen-activated protein kinase) and interfered with postsynaptic toxic signaling complexes engaged by Abeta.
Depletion of p38gamma increased the severity of neuronal circuit aberrations, cognitive deficits, and premature lethality in a mouse model of AD, whereas increasing the activity of p38gamma abolished these deficits. Furthermore, mimicking site-specific tau phosphorylation alleviated Abeta-induced neuronal death and offered protection from its toxicity.
"This study has completely changed our understanding of what happens in the brain during the development of Alzheimer's disease," said senior author Dr. Lars Ittner, professor of medicine at the University of New South Wales. "Amyloid-beta induces toxicity in the neurons but the first step in tau phosphorylation is actually to decrease this toxicity. This is a completely new mindset; that the reason tau becomes modified is actually to protect from damage. We found that p38gamma, which initially offers protection, fades away early in the brains of people with AD, suggesting a loss of protection."
"We set out to find mediators of this progression, which led us quickly to our surprising finding. It was the opposite of what we expected. It was only when we changed our view of the process involved in the development of AD that these results started to make sense," said Dr. Ittner. "We used mice to screen for a very specific toxicity that we knew from previous work is involved in the progression of the disease. Part of our study involved reintroducing p38gamma and increasing its activity. We saw that, in mice, it could prevent memory deficits from happening, so it has true therapeutic potential. If we can stimulate that activity, we may be able to delay or even halt the progression of Alzheimer's disease."
Related Links:
University of New South Wales










 Analyzer.jpg)