Stabilizing Iron-Sulfur Transport Clusters Reduces Size and Aggressiveness of Tumors
By LabMedica International staff writers
Posted on 21 Sep 2016
An international team of cancer researchers has identified a protein that, when overexpressed, enabled breast tumors to better withstand oxidative stress thereby becoming much larger and more aggressive.Posted on 21 Sep 2016
The protein in question is the iron–sulfur (Fe-S) nutrient-deprivation autophagy factor-1 (NAF-1) protein, a member of the NEET family that transport clusters of iron and sulfur molecules inside cells. The clusters, which adhere to the mitochondrial membrane, help regulate processes in cells by controlling reduction-oxidation (redox) and metabolic activity. NAF-1 is unique among Fe-S proteins due to its 3Cys-1His cluster coordination structure that allows it to be relatively stable, while still being able to transfer its clusters to apo-acceptor proteins.
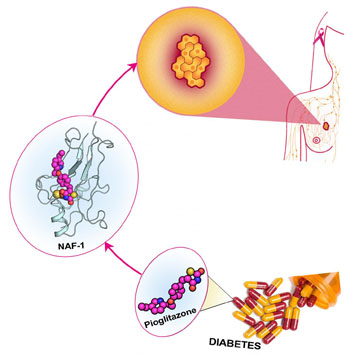
Image: Researchers have discovered that the drug pioglitazone, used to treat diabetes, shows some ability to halt the overexpression of the protein NAF-1, which has been associated with the proliferation of breast cancer (Photo courtesy of Fang Bai, Rice University).
Investigators at The Hebrew University of Jerusalem (Israel) and Rice University (Houston, TX, USA) reported in the September 12, 2016, online edition of the journal Proceedings of the [U.S.] National Academy of Sciences that overexpression of NAF-1 in xenograft breast cancer tumors resulted in a dramatic augmentation in tumor size and aggressiveness, and that NAF-1 overexpression enhanced the tolerance of cancer cells to oxidative stress.
The importance of NAF-1 overexpression was emphasized by the discovery of a NAF-1 mutant with a single point mutation that stabilized the NAF-1 clusters. The presence of the mutated form of the protein in xenograft breast cancer tumors resulted in a dramatic decrease in tumor size that was accompanied by enhanced mitochondrial iron and reactive oxygen accumulation and reduced cellular tolerance to oxidative stress. Treating breast cancer cells with pioglitazone, a compound that stabilized the 3Cys-1His cluster of NAF-1, resulted in a similar effect on mitochondrial iron and reactive oxygen species accumulation. This finding supports the potential use of drugs that suppress NAF-1 accumulation or stabilize its clusters for the treatment of cancers that display high expression levels of NAF-1.
Senior author Dr. Rachel Nechushtai, professor of biochemistry at The Hebrew University of Jerusalem, said, "Tumors depend on the lability, or the transient nature, of the clusters. The more NAF-1 you make, and the more its clusters can be transferred, the bigger the tumor develops. We knew from previous studies that pioglitazone stabilizes the cluster. With the mutant, we hardly got any tumors and did not see angiogenesis (the process through which new blood vessels form). When we did see tumors, they were white, not red, because they had no blood vessels."
"We thought, "How do we connect this to the clinics?" The only connection was to try a drug that, like the mutation, also stabilizes the cluster," said Dr. Nechushtai. "Dr. Fang Bai of Rice University showed in her simulations where the binding site is and why the drug stabilizes the cluster."
Related Links:
Hebrew University of Jerusalem
Rice University














