Mechanical Stress Modifies Growth of Tumor Cultures and Response to Chemotherapeutic Agents
By LabMedica International staff writers
Posted on 19 Aug 2015
A more effective method of assessing the response of tumors to chemotherapeutic drugs utilizes three-dimensional culture growth combined with the application of mechanical stress factors that the tumor would experience in vivo.Posted on 19 Aug 2015
Investigators at Rice University (Houston, TX, USA) and the University of Texas MD Anderson Cancer Center (Houston, USA) developed a method for culturing Ewing sarcoma (ES) cells on three-dimensional scaffolds within a flow perfusion bioreactor, which provided mechanical stimulation by changing the fluid viscosity and flow rate to induce flow-derived shear stress.
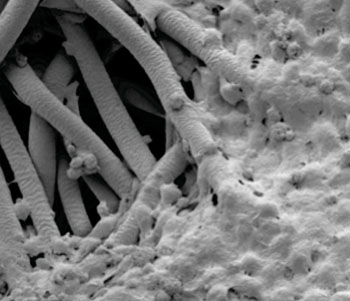
Image: Electron micrograph showing bone cancer cells populating the surface of a bioscaffold that had been placed in a flow perfusion bioreactor to evaluate the cells' response to the mechanical forces they experience in the body (Photo courtesy of the University of Texas MD Anderson Cancer Center and Rice University).
Results published in the August 3, 2015, edition of the journal Proceedings of the National Academy of Sciences of the United States of America (PNAS) revealed that in the 10 day study, the steady flow of fluid through the scaffold prompted the sarcoma cells to proliferate throughout the structure. Shear stress induced the cells to significantly increase their production of IGF-1 protein and also down-regulated the production of two other cancer-related proteins, c-KIT and HER2, compared with tumors grown in static two-dimensional cultures.
This finding was particularly relevant, given the central role of the IGF-1/IGF-1 receptor (IGF-1R) pathway in ES tumorigenesis and as a promising clinical target. The insulin-like growth factors (IGFs) are proteins with high sequence similarity to insulin. IGFs are part of a complex system that cells use to communicate with their physiologic environment. This complex system consists of two cell-surface receptors (IGF-IR and IGF-IIR), two ligands (IGF-I and IGF-II), a family of six high-affinity IGF-binding proteins (IGFBP 1-6), as well as associated IGFBP degrading enzymes.
The use of a tissue-engineered model for this study, rather than human tumors or xenografts, enabled precise control of the forces experienced by the ES cells. This controlled environment enabled the observation that flow perfusion enhanced, in a rate-dependent manner, the sensitivity of ES cells to dalotuzumab, a drug that disrupts the IGF-1 pathway. The conditions used in this study allowed the investigators to demonstrate the shear stress-dependent ES cell sensitivity to dalotuzumab, highlighting the importance of biomechanical stimulation on ES-acquired drug resistance to IGF-1R inhibition.
"Mechanical forces are present in our bodies even though we are not always aware of them," said first author Dr. Marco Santoro, a graduate researcher in chemical and biomolecular engineering at The University of Texas MD Anderson Cancer Center. "Our cells are sensitive to the forces around them and change their behavior accordingly. Tumor cells behave the same way, changing their function depending on the forces they sense. For the first time, we showed how the effect of the drug changes according to the forces experienced by the cells. IGF-1 is crucial for this kind of sarcoma, which relies on this mechanism for growth. We show that the higher the mechanical stimulation, the more pronounced the secretion of this particular protein."
Related Links:
Rice University
University of Texas MD Anderson Cancer Center








 Analyzer.jpg)


 (3) (1).png)


