New Version of Old Drug Shows Promise for Treating Drug-Resistant Tuberculosis
By LabMedica International staff writers
Posted on 16 Jun 2015
A team of molecular microbiologists has determined the mechanism by which the Streptomyces-derived antibiotic griselimycin blocks the growth of Mycobacterium tuberculosis, the bacterium responsible for causing more than eight million cases of tuberculosis annually on a worldwide basis.Posted on 16 Jun 2015
Investigators from the Helmholtz Center for Infection Research (Braunschweig, Germany), other German research institutes, and the biomedical company Sanofi (Paris, France) were interested in exploring the possibility of using griselimycin or one of its derivatives for treating drug resistant tuberculosis; while this drug had been evaluated in the 1960's it had suffered in comparison to others. However, M. tuberculosis has developed resistance to most of those other drugs, and development of replacements is a top priority.
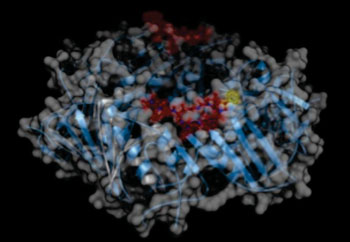
Image: DNA polymerase forms a homodimeric ring (shown as blue cartoon and surface representation). Each polypeptide chain binds one molecule of griselimycin (red). The optimized compound cyclohexylgriselimycin contains an additional cyclohexane moiety (yellow, shown only for the ligand in the foreground) (Photo courtesy of the Helmholtz Centre for Infection Research).
The investigators reported in the June 5, 2015, issue of the journal Science that a variant of griselimycinm, cyclohexylgriselimycin, was particularly effective against M. tuberculosis, in cells and when administered orally to an animal model.
The effectiveness of cyclohexylgriselimycin was found to be due to the drug's inhibition of the M. tuberculosis DNA polymerase sliding clamp DnaN. A DNA clamp, also known as a sliding clamp, is a protein fold that serves as a processivity-promoting factor in DNA replication. Processivity is an enzyme's ability to catalyze consecutive reactions without releasing its substrate. As a critical component of the DNA polymerase III holoenzyme, the clamp protein binds DNA polymerase and prevents this enzyme from dissociating from the template DNA strand. The clamp-polymerase protein–protein interactions are stronger and more specific than the direct interactions between the polymerase and the template DNA strand; because one of the rate-limiting steps in the DNA synthesis reaction is the association of the polymerase with the DNA template, the presence of the sliding clamp dramatically increases the number of nucleotides that the polymerase can add to the growing strand per association event. The presence of the DNA clamp can increase the rate of DNA synthesis up to 1,000-fold compared with a nonprocessive polymerase.
As inhibiting the DNA clamp is a completely different mechanisms from those of antibiotics now used against tuberculosis and other bacterial pathogens, the investigators consider that the risk of developing resistance to cyclohexylgriselimycin is low.
"We hope that cyclohexylgriselimycin will become an agent that can even be used against resistant tuberculosis pathogens in the future and contributes to a more successful fight against this dreadful disease," said senior author Dr. Rolf Müller, head of the department of microbial natural products at the Helmholtz Centre for Infection Research. "In the tuberculosis pathogen, the substance binds to the so-called DNA clamp and thus suppresses the activity of the DNA polymerase enzyme, which multiplies the genetic information inside the cell. We resumed the work on this agent and optimized it such that it shows excellent activity in the infection model—even against multi-resistant tuberculosis pathogens."
Related Links:
Helmholtz Centre for Infection Research
Sanofi














