Mesa Biotech Launches Molecular Test System at AACC 2019
By LabMedica International staff writers
Posted on 06 Aug 2019
Mesa Biotech Inc. (San Diego, CA, USA), a privately-held, molecular diagnostic company that has developed an affordable, sample-to-answer, CLIA-waived PCR (polymerase chain reaction) testing platform designed specifically for point-of-care (POC) infectious disease diagnosis, launched its respiratory syncytial virus test (RSV) at AACC 2019. Mesa demonstrated its expanded, novel Accula Test System at the 71st American Association of Clinical Chemistry (AACC) Annual Meeting and Clinical Lab Expo held on August 4-8, 2019, at the convention center in Anaheim, CA, USA.Posted on 06 Aug 2019
Mesa designs, develops, manufactures and commercializes next-generation molecular diagnostic tests, bringing the superior diagnostic performance of nucleic acid PCR amplification to the POC. Mesa’s Accula System is a sample-to-answer palm-sized, reusable dock with single-use test cassettes. The novel molecular test system is a visually interpreted, FDA-cleared, CLIA-waived PCR platform with the simplicity, convenience and procedural familiarity of traditional POC rapid immunoassay tests, while providing the superior sensitivity, specificity and information content of laboratory-based PCR testing.
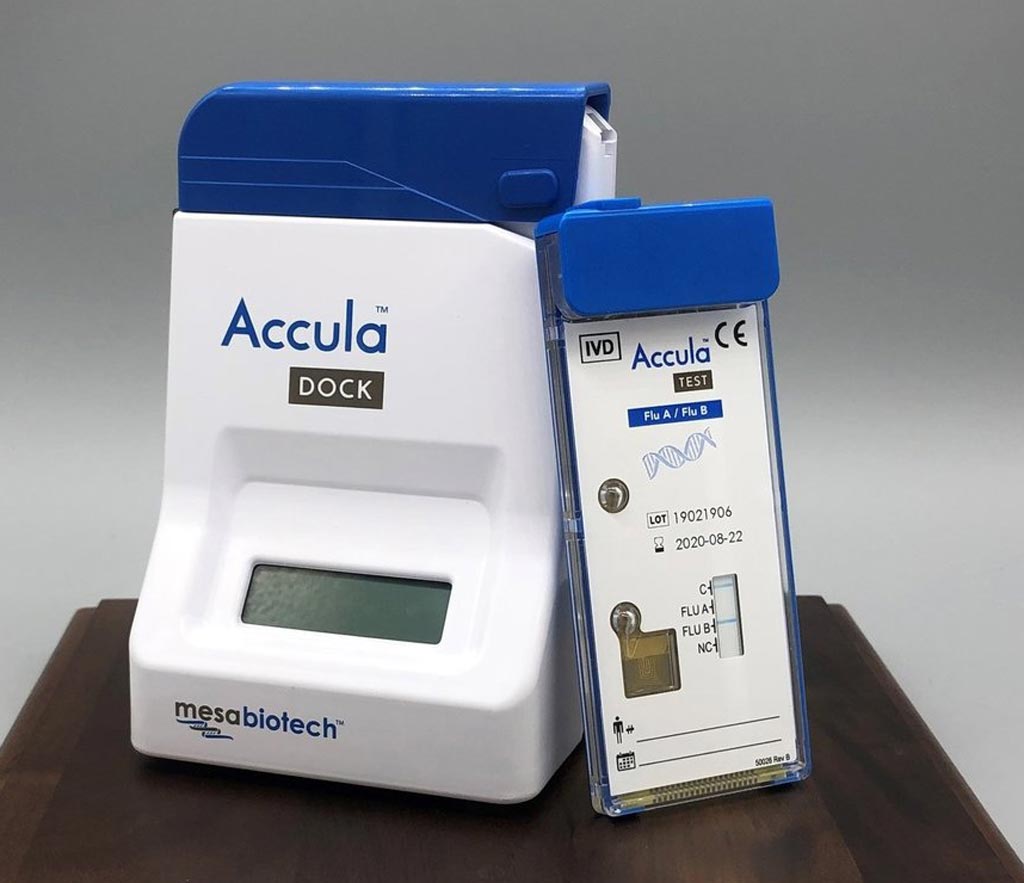
Image: The Accula system for POC testing (Photo courtesy of Mesa Biotech).
At AACC 2019, Mesa exhibited the Accula System's RSV and Flu A/Flu B molecular tests, which are distributed by Sekisui Diagnostics in the U.S. under the Silaris brand. Both the Accula Flu A/Flu B and RSV tests are indicated for use with nasal swab collection that is less invasive than nasopharyngeal swabs and allows for a more comfortable specimen collection experience for the patient.
"We are excited to launch our second Accula point-of-care test at AACC," said Hong Cai, Co-founder and Chief Executive Officer, Mesa Biotech, Inc. "Similar to influenza, RSV requires a prompt and accurate diagnosis at the POC so treatment can begin, making our RSV test an important addition to our sample-to-answer PCR testing platform."
Related Links:
Mesa Biotech