A Focus on Advocacy — From Washington to Chicago
|
By Evan Fortman (AACC) Posted on 12 Jul 2022 |

Image: A Focus on Advocacy — From Washington to Chicago (Photo courtesy of aacc.org)
Throughout 2022, AACC’s primary advocacy focus has been on preventing the passage of the Verifying Accurate Leading-edge IVCT Development (VALID) Act, which would significantly alter the regulation of laboratory developed tests (LDTs). If enacted, VALID would create new duplicative FDA requirements for laboratories that perform LDTs on top of the already stringent regulatory framework overseen by the Centers for Medicare and Medicaid Services (CMS) through the Clinical Laboratory Improvement Amendments (CLIA).
Supporters of VALID have been making an effort to fast-track the legislation by attaching it to the FDA Safety and Landmark Advancements (FDASLA) Act - a bill that, among other things, would reauthorize the Food and Drug Administration’s user-fee amendments for the next five years. This push has been bolstered by ostensibly well meaning, yet misleading information about the regulation of LDTs that the association has worked diligently to correct. AACC has written and joined other stakeholders in several letters urging Congress to address the healthcare community’s concerns with the legislation prior to advancing VALID. To garner further attention on Capitol Hill, AACC conducted an ad campaign and submitted an open letter through Politico.
Throughout the spring, AACC members have met virtually with the offices of legislators on the Senate Health, Labor Education, Pensions (HELP) Committee, and the House Energy & Commerce Committee, both of which are currently considering the legislation. In April, AACC joined the Association for Molecular Pathology (AMP), American College of Medical Genetics (ACMG), and the Association of Pathology Chairs (APC) for a briefing to educate congressional staff on the significant negative impact the VALID Act would have on the practice of medicine by limiting labs' ability to perform LDTs.
Critically, many members have participated in the grassroots campaign by sending letters to their legislators detailing the impact VALID would have on the ability of their organizations to conduct cutting-edge testing for the patient populations they serve.
AACC along with a broad array of healthcare stakeholders believe that the complexity of VALID, and its potential harm to innovation and patient care, require Congress to provide ample time for thorough consideration and stakeholder engagement to avoid unintended consequences that may result from passage of the legislation.
Looking to learn more about regulation of LDTs during the AACC Annual Scientific Meeting in Chicago? A scientific session on July 25 will cover the legislation Congress has introduced and the potential impact on laboratory medicine. The session, “VALID, VITAL, LDT” will be led by experts with experience in both advocacy and LDTs in the lab.
Supporters of VALID have been making an effort to fast-track the legislation by attaching it to the FDA Safety and Landmark Advancements (FDASLA) Act - a bill that, among other things, would reauthorize the Food and Drug Administration’s user-fee amendments for the next five years. This push has been bolstered by ostensibly well meaning, yet misleading information about the regulation of LDTs that the association has worked diligently to correct. AACC has written and joined other stakeholders in several letters urging Congress to address the healthcare community’s concerns with the legislation prior to advancing VALID. To garner further attention on Capitol Hill, AACC conducted an ad campaign and submitted an open letter through Politico.
Throughout the spring, AACC members have met virtually with the offices of legislators on the Senate Health, Labor Education, Pensions (HELP) Committee, and the House Energy & Commerce Committee, both of which are currently considering the legislation. In April, AACC joined the Association for Molecular Pathology (AMP), American College of Medical Genetics (ACMG), and the Association of Pathology Chairs (APC) for a briefing to educate congressional staff on the significant negative impact the VALID Act would have on the practice of medicine by limiting labs' ability to perform LDTs.
Critically, many members have participated in the grassroots campaign by sending letters to their legislators detailing the impact VALID would have on the ability of their organizations to conduct cutting-edge testing for the patient populations they serve.
AACC along with a broad array of healthcare stakeholders believe that the complexity of VALID, and its potential harm to innovation and patient care, require Congress to provide ample time for thorough consideration and stakeholder engagement to avoid unintended consequences that may result from passage of the legislation.
Looking to learn more about regulation of LDTs during the AACC Annual Scientific Meeting in Chicago? A scientific session on July 25 will cover the legislation Congress has introduced and the potential impact on laboratory medicine. The session, “VALID, VITAL, LDT” will be led by experts with experience in both advocacy and LDTs in the lab.
Latest AACC 2022 News
- AACC 2022 Review: International Meetings Rebound After Suffering Long COVID Hiatus
- Faster Method Diagnoses Pediatric Urinary Tract Infections
- Tianlong Showcases Integrated PCR Lab Solutions at AACC 2022
- Cellavision Introduces New Workflow Solution for Low-Volume Hematology Labs at AACC 2022
- Advanced Instruments Introduces New Automated Osmometer at AACC 2022
- Innovative Smartphone and AI-Based Tests Featured at AACC 2022
- New Tests Presented at AACC 2022 to Solve Challenges in Children's Healthcare
- 10-Minute Sepsis Risk Stratification Test Introduced at AACC 2022
- New System Combining DNA/RNA Extraction and Smart PCR Setup Launched at AACC 2022
- Novel Small Volume Blood Collection Device Launched at AACC 2022
- AACC Disruptive Technology Award Finalists Tackle Cancer, Women's Health, and STDs
- Meridian Demonstrates State-of-the-Art Urea Breath Test System at AACC 2022
- Nikon Instruments Displays Latest Clinical Pathology Products at AACC 2022
- Seegene Showcases Latest Molecular Testing Solutions at AACC 2022
- Werfen Introduces New Acute Care Diagnostic Products for CVOR at AACC 2022
- PerkinElmer Demonstrates Vanadis NIPT Screening Platform Featuring Groundbreaking cfDNA Technology
Channels
Clinical Chemistry
view channel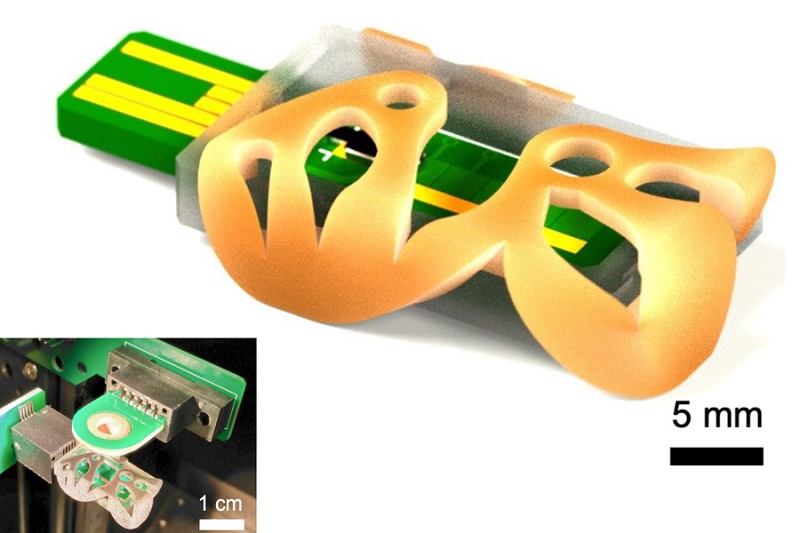
3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
Mass spectrometry is a precise technique for identifying the chemical components of a sample and has significant potential for monitoring chronic illness health states, such as measuring hormone levels... Read more.jpg)
POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
Exosomes, tiny cellular bioparticles carrying a specific set of proteins, lipids, and genetic materials, play a crucial role in cell communication and hold promise for non-invasive diagnostics.... Read more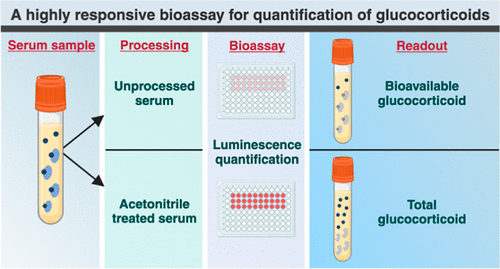
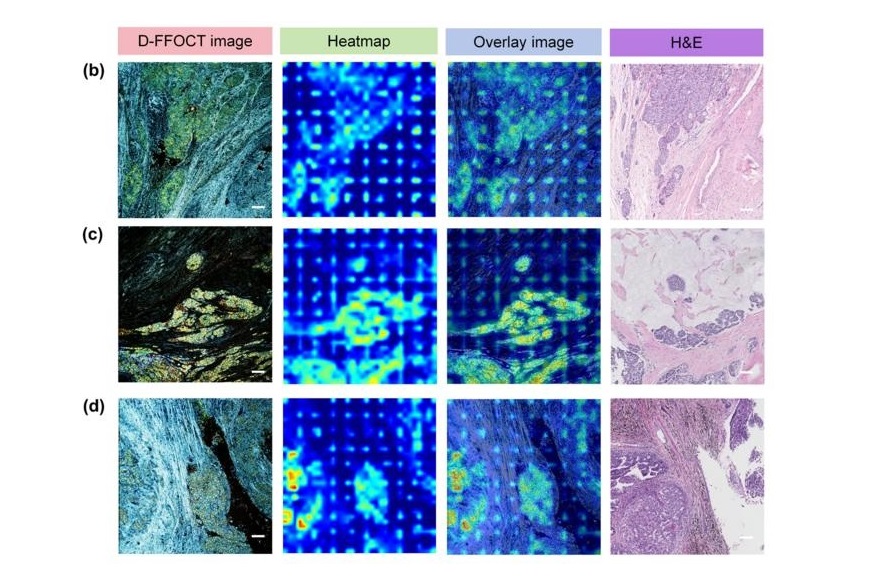
Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
The conventional methods for measuring free cortisol, the body's stress hormone, from blood or saliva are quite demanding and require sample processing. The most common method, therefore, involves collecting... Read moreMolecular Diagnostics
view channel
World’s First One-Minute Hepatitis C Antibody Test Facilitates Quick Triage
Rapid and accurate testing is crucial in the fight against Hepatitis C. Hepatitis C blood testing determines whether someone has been infected with the Hepatitis C virus. Having regular access to testing... Read more
Game-Changing Blood Test for Stroke Detection Could Bring Life-Saving Care to Patients
Stroke is the primary cause of disability globally and ranks as the second leading cause of death. However, timely early intervention can prevent severe outcomes. Most strokes are ischemic, resulting from... Read moreHematology
view channel
Next Generation Instrument Screens for Hemoglobin Disorders in Newborns
Hemoglobinopathies, the most widespread inherited conditions globally, affect about 7% of the population as carriers, with 2.7% of newborns being born with these conditions. The spectrum of clinical manifestations... Read more
First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
Arboviruses represent an emerging global health threat, exacerbated by climate change and increased international travel that is facilitating their spread across new regions. Chikungunya, dengue, West... Read more
POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
Neutropenia, a decrease in neutrophils (a type of white blood cell crucial for fighting infections), is a frequent side effect of certain cancer treatments. This condition elevates the risk of infections,... Read more
First Affordable and Rapid Test for Beta Thalassemia Demonstrates 99% Diagnostic Accuracy
Hemoglobin disorders rank as some of the most prevalent monogenic diseases globally. Among various hemoglobin disorders, beta thalassemia, a hereditary blood disorder, affects about 1.5% of the world's... Read moreImmunology
view channel.jpg)
AI Predicts Tumor-Killing Cells with High Accuracy
Cellular immunotherapy involves extracting immune cells from a patient's tumor, potentially enhancing their cancer-fighting capabilities through engineering, and then expanding and reintroducing them into the body.... Read more
Diagnostic Blood Test for Cellular Rejection after Organ Transplant Could Replace Surgical Biopsies
Transplanted organs constantly face the risk of being rejected by the recipient's immune system which differentiates self from non-self using T cells and B cells. T cells are commonly associated with acute... Read more
AI Tool Precisely Matches Cancer Drugs to Patients Using Information from Each Tumor Cell
Current strategies for matching cancer patients with specific treatments often depend on bulk sequencing of tumor DNA and RNA, which provides an average profile from all cells within a tumor sample.... Read more
Genetic Testing Combined With Personalized Drug Screening On Tumor Samples to Revolutionize Cancer Treatment
Cancer treatment typically adheres to a standard of care—established, statistically validated regimens that are effective for the majority of patients. However, the disease’s inherent variability means... Read moreMicrobiology
view channel
Integrated Solution Ushers New Era of Automated Tuberculosis Testing
Tuberculosis (TB) is responsible for 1.3 million deaths every year, positioning it as one of the top killers globally due to a single infectious agent. In 2022, around 10.6 million people were diagnosed... Read more
Automated Sepsis Test System Enables Rapid Diagnosis for Patients with Severe Bloodstream Infections
Sepsis affects up to 50 million people globally each year, with bacteraemia, formerly known as blood poisoning, being a major cause. In the United States alone, approximately two million individuals are... Read moreEnhanced Rapid Syndromic Molecular Diagnostic Solution Detects Broad Range of Infectious Diseases
GenMark Diagnostics (Carlsbad, CA, USA), a member of the Roche Group (Basel, Switzerland), has rebranded its ePlex® system as the cobas eplex system. This rebranding under the globally renowned cobas name... Read more
Clinical Decision Support Software a Game-Changer in Antimicrobial Resistance Battle
Antimicrobial resistance (AMR) is a serious global public health concern that claims millions of lives every year. It primarily results from the inappropriate and excessive use of antibiotics, which reduces... Read morePathology
view channel
Groundbreaking CRISPR Screen Technology Rapidly Determines Disease Mechanism from Tissues
Thanks to over a decade of advancements in human genetics, scientists have compiled extensive lists of genetic variations linked to a wide array of human diseases. However, understanding how a gene contributes... Read more
New AI Tool Classifies Brain Tumors More Quickly and Accurately
Precision in diagnosing and categorizing tumors is essential for delivering effective treatment to patients. Currently, the gold standard for identifying various types of brain tumors involves DNA methylation-based... Read moreTechnology
view channel
New Diagnostic System Achieves PCR Testing Accuracy
While PCR tests are the gold standard of accuracy for virology testing, they come with limitations such as complexity, the need for skilled lab operators, and longer result times. They also require complex... Read more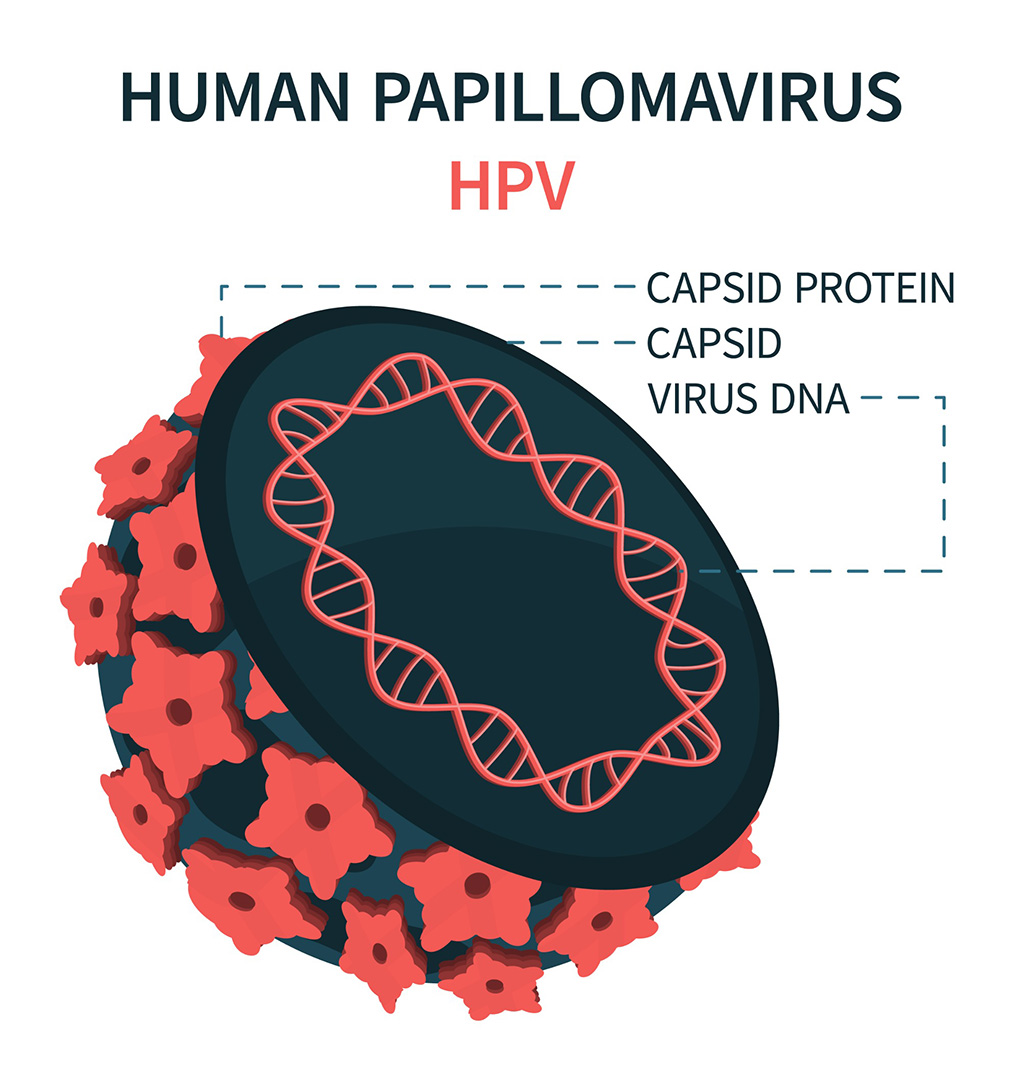
DNA Biosensor Enables Early Diagnosis of Cervical Cancer
Molybdenum disulfide (MoS2), recognized for its potential to form two-dimensional nanosheets like graphene, is a material that's increasingly catching the eye of the scientific community.... Read more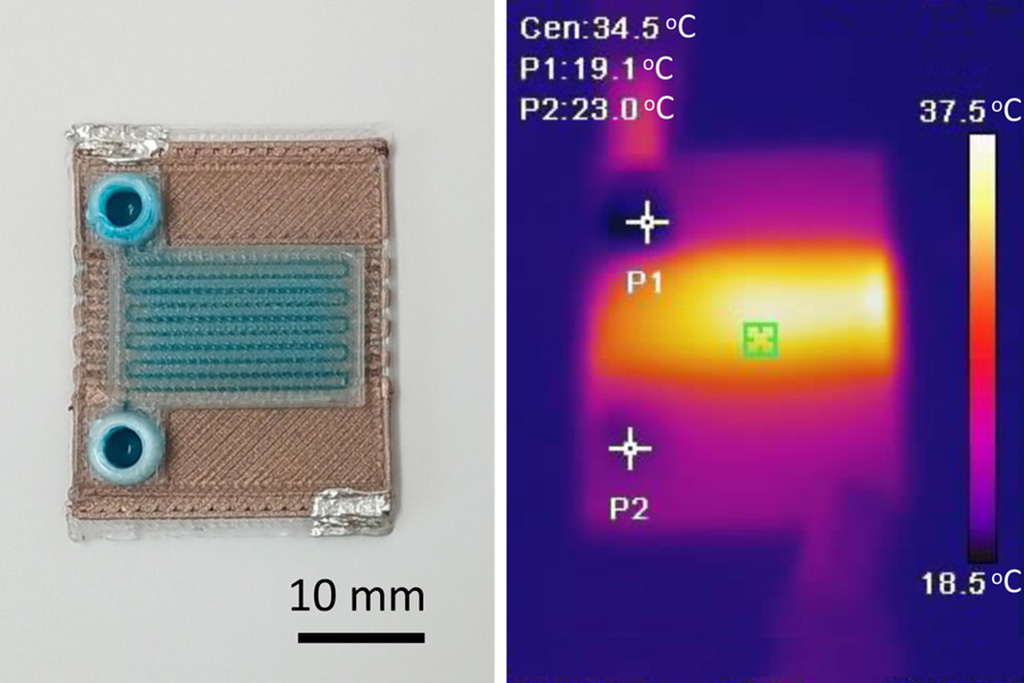
Self-Heating Microfluidic Devices Can Detect Diseases in Tiny Blood or Fluid Samples
Microfluidics, which are miniature devices that control the flow of liquids and facilitate chemical reactions, play a key role in disease detection from small samples of blood or other fluids.... Read more
Breakthrough in Diagnostic Technology Could Make On-The-Spot Testing Widely Accessible
Home testing gained significant importance during the COVID-19 pandemic, yet the availability of rapid tests is limited, and most of them can only drive one liquid across the strip, leading to continued... Read moreIndustry
view channel
Danaher and Johns Hopkins University Collaborate to Improve Neurological Diagnosis
Unlike severe traumatic brain injury (TBI), mild TBI often does not show clear correlations with abnormalities detected through head computed tomography (CT) scans. Consequently, there is a pressing need... Read more
Beckman Coulter and MeMed Expand Host Immune Response Diagnostics Partnership
Beckman Coulter Diagnostics (Brea, CA, USA) and MeMed BV (Haifa, Israel) have expanded their host immune response diagnostics partnership. Beckman Coulter is now an authorized distributor of the MeMed... Read more_1.jpg)