Randox Highlights Its Innovative Diagnostic Products Revolutionizing Healthcare at MEDLAB Middle East
|
By LabMedica International staff writers Posted on 25 Jan 2022 |

Randox Laboratories (Crumlin, UK) showcased the Vivalytic all-in-one molecular diagnostics solution and the Randox Discovery benchtop lab at MEDLAB Middle East which took place 24-27 January 2022 at the Dubai World Trade Centre.
The innovative platform Vivalytic developed by Randox in partnership with Bosch aims to change accessibility to molecular diagnostics. Using Randox-patented Biochip Array Technology, it is the easiest-to-use and most-comprehensive multiplex PCR platform on the market. It provides the broadest range of test options ever seen for an analyzer of its size, and also supports single-plex and low-plex testing, simplifying the processes for otherwise-complex laboratory test procedures. Depending on the test application, results will be delivered from 30 minutes. The Vivalytic is the perfect fit for any laboratory with numerous benefits to enhance testing capabilities.
Also on display at this year’s at MEDLAB Middle East was the Randox Discovery, an exciting and unique analyzer consolidating molecular and immunoassay workflows onto one compact benchtop platform. The COVID-19 diagnostic analyzer is the first to combine sample preparation techniques including extraction, PCR & Biochip Technology. Simple and easy to use, the intuitive user-interface guides the operator through the entire testing process. Built with the user and laboratory in mind, a single operator is all that is required to run up to three fully automated discovery platforms increasing operator walkaway time and ensuring rapid turnaround time of three hours to first batch results.
Related Links:
Randox Laboratories
Latest Medlab 2022 News
- EUROIMMUN Presents EUROPattern Microscope Live with EUROLabOffice 4.0 Software for Ultrafast Automated Microscopy
- PerkinElmer Exhibits Automated Nucleic Acid Extraction Solutions at MEDLAB Middle East
- Abbott Displays Its Life-Changing POC Tests and Diagnostic Tools at MEDLAB Middle East
- Diagast Presents Latest Immunohaematology Solutions at MEDLAB Middle East
- Siemens Brings Its Latest Innovations in Laboratory Diagnostics to MEDLAB Middle East 2022
- Bioperfectus Showcases Its Molecular Diagnostic Products for Infectious Diseases at MEDLAB 2022 Online
- Seegene Showcases New STARlet-AIOS All-in-One Solution for All Molecular Testing at MEDLAB Middle East
- LumiraDx Presents Its Next-Generation POC Diagnostics Platform and Fast Lab Solutions at MEDLAB Middle East
- Beckman Coulter Demonstrates Newly Launched DxA 5000 Fit Workflow Automation System at MEDLAB Middle East
- Horiba Presents Comprehensive Range of Hematology, Hemostasis and Clinical Chemistry Analyzers at MEDLAB Middle East
- MGI Tech Launches New MGISP-Smart 8 Automated Sample Preparation System at MEDLAB Middle East
- GC Labs Highlight Its “Smart Lab” Concept at MEDLAB Middle East 2022
- MEDLAB Middle East and Arab Health 2022 Edition Return as Co-Located Events for Healthcare and Laboratory Industries
Channels
Clinical Chemistry
view channel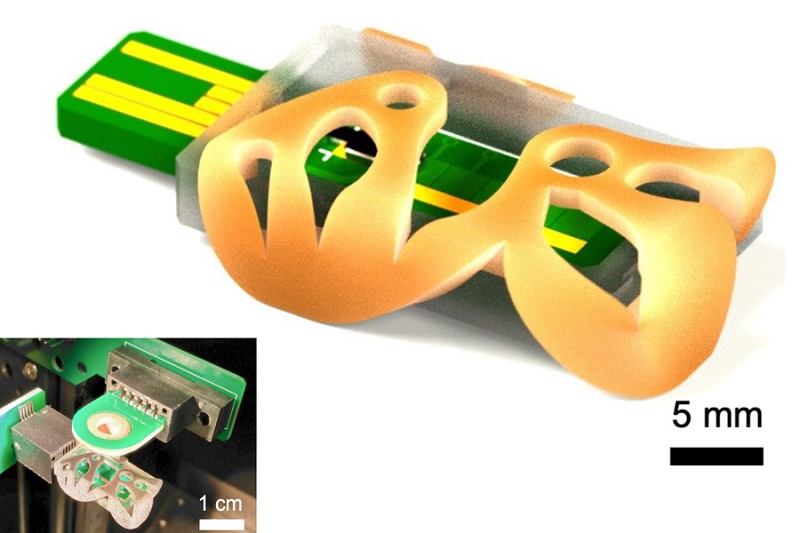
3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
Mass spectrometry is a precise technique for identifying the chemical components of a sample and has significant potential for monitoring chronic illness health states, such as measuring hormone levels... Read more.jpg)
POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
Exosomes, tiny cellular bioparticles carrying a specific set of proteins, lipids, and genetic materials, play a crucial role in cell communication and hold promise for non-invasive diagnostics.... Read more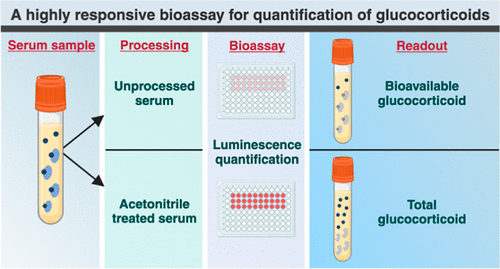
Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
The conventional methods for measuring free cortisol, the body's stress hormone, from blood or saliva are quite demanding and require sample processing. The most common method, therefore, involves collecting... Read moreMolecular Diagnostics
view channel
New Genetic Testing Procedure Combined With Ultrasound Detects High Cardiovascular Risk
A key interest area in cardiovascular research today is the impact of clonal hematopoiesis on cardiovascular diseases. Clonal hematopoiesis results from mutations in hematopoietic stem cells and may lead... Read more
Blood Samples Enhance B-Cell Lymphoma Diagnostics and Prognosis
B-cell lymphoma is the predominant form of cancer affecting the lymphatic system, with about 30% of patients with aggressive forms of this disease experiencing relapse. Currently, the disease’s risk assessment... Read moreHematology
view channel
Next Generation Instrument Screens for Hemoglobin Disorders in Newborns
Hemoglobinopathies, the most widespread inherited conditions globally, affect about 7% of the population as carriers, with 2.7% of newborns being born with these conditions. The spectrum of clinical manifestations... Read more
First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
Arboviruses represent an emerging global health threat, exacerbated by climate change and increased international travel that is facilitating their spread across new regions. Chikungunya, dengue, West... Read more
POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
Neutropenia, a decrease in neutrophils (a type of white blood cell crucial for fighting infections), is a frequent side effect of certain cancer treatments. This condition elevates the risk of infections,... Read more
First Affordable and Rapid Test for Beta Thalassemia Demonstrates 99% Diagnostic Accuracy
Hemoglobin disorders rank as some of the most prevalent monogenic diseases globally. Among various hemoglobin disorders, beta thalassemia, a hereditary blood disorder, affects about 1.5% of the world's... Read moreImmunology
view channel
Diagnostic Blood Test for Cellular Rejection after Organ Transplant Could Replace Surgical Biopsies
Transplanted organs constantly face the risk of being rejected by the recipient's immune system which differentiates self from non-self using T cells and B cells. T cells are commonly associated with acute... Read more
AI Tool Precisely Matches Cancer Drugs to Patients Using Information from Each Tumor Cell
Current strategies for matching cancer patients with specific treatments often depend on bulk sequencing of tumor DNA and RNA, which provides an average profile from all cells within a tumor sample.... Read more
Genetic Testing Combined With Personalized Drug Screening On Tumor Samples to Revolutionize Cancer Treatment
Cancer treatment typically adheres to a standard of care—established, statistically validated regimens that are effective for the majority of patients. However, the disease’s inherent variability means... Read moreMicrobiology
view channel
Clinical Decision Support Software a Game-Changer in Antimicrobial Resistance Battle
Antimicrobial resistance (AMR) is a serious global public health concern that claims millions of lives every year. It primarily results from the inappropriate and excessive use of antibiotics, which reduces... Read more
New CE-Marked Hepatitis Assays to Help Diagnose Infections Earlier
According to the World Health Organization (WHO), an estimated 354 million individuals globally are afflicted with chronic hepatitis B or C. These viruses are the leading causes of liver cirrhosis, liver... Read more
1 Hour, Direct-From-Blood Multiplex PCR Test Identifies 95% of Sepsis-Causing Pathogens
Sepsis contributes to one in every three hospital deaths in the US, and globally, septic shock carries a mortality rate of 30-40%. Diagnosing sepsis early is challenging due to its non-specific symptoms... Read morePathology
view channel.jpg)
Use of DICOM Images for Pathology Diagnostics Marks Significant Step towards Standardization
Digital pathology is rapidly becoming a key aspect of modern healthcare, transforming the practice of pathology as laboratories worldwide adopt this advanced technology. Digital pathology systems allow... Read more
First of Its Kind Universal Tool to Revolutionize Sample Collection for Diagnostic Tests
The COVID pandemic has dramatically reshaped the perception of diagnostics. Post the pandemic, a groundbreaking device that combines sample collection and processing into a single, easy-to-use disposable... Read moreAI-Powered Digital Imaging System to Revolutionize Cancer Diagnosis
The process of biopsy is important for confirming the presence of cancer. In the conventional histopathology technique, tissue is excised, sliced, stained, mounted on slides, and examined under a microscope... Read more
New Mycobacterium Tuberculosis Panel to Support Real-Time Surveillance and Combat Antimicrobial Resistance
Tuberculosis (TB), the leading cause of death from an infectious disease globally, is a contagious bacterial infection that primarily spreads through the coughing of patients with active pulmonary TB.... Read moreTechnology
view channel
New Diagnostic System Achieves PCR Testing Accuracy
While PCR tests are the gold standard of accuracy for virology testing, they come with limitations such as complexity, the need for skilled lab operators, and longer result times. They also require complex... Read more
DNA Biosensor Enables Early Diagnosis of Cervical Cancer
Molybdenum disulfide (MoS2), recognized for its potential to form two-dimensional nanosheets like graphene, is a material that's increasingly catching the eye of the scientific community.... Read more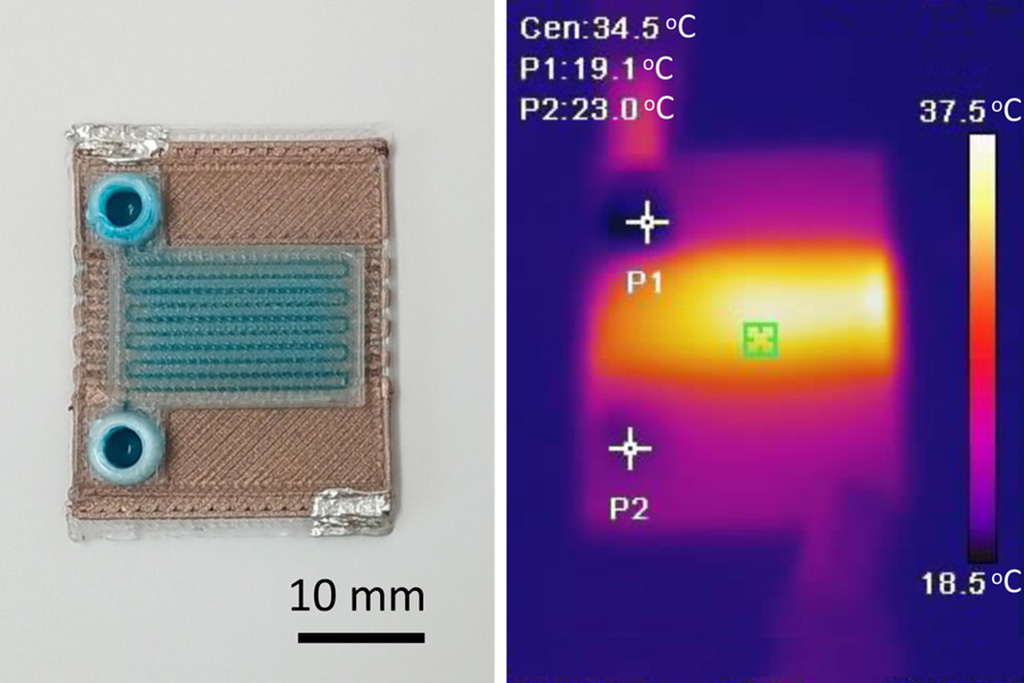
Self-Heating Microfluidic Devices Can Detect Diseases in Tiny Blood or Fluid Samples
Microfluidics, which are miniature devices that control the flow of liquids and facilitate chemical reactions, play a key role in disease detection from small samples of blood or other fluids.... Read more
Breakthrough in Diagnostic Technology Could Make On-The-Spot Testing Widely Accessible
Home testing gained significant importance during the COVID-19 pandemic, yet the availability of rapid tests is limited, and most of them can only drive one liquid across the strip, leading to continued... Read moreIndustry
view channel_1.jpg)
Thermo Fisher and Bio-Techne Enter Into Strategic Distribution Agreement for Europe
Thermo Fisher Scientific (Waltham, MA USA) has entered into a strategic distribution agreement with Bio-Techne Corporation (Minneapolis, MN, USA), resulting in a significant collaboration between two industry... Read more
ECCMID Congress Name Changes to ESCMID Global
Over the last few years, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID, Basel, Switzerland) has evolved remarkably. The society is now stronger and broader than ever before... Read more