Whole Blood Rapid Test Aids Assessment of Concussion at Patient's Bedside
Posted on 02 Apr 2024
In the United States annually, approximately five million individuals seek emergency department care for traumatic brain injuries (TBIs), yet over half of those suspecting a concussion may never get it checked. Traditional TBI assessments, including the Glasgow Coma Scale, subjective evaluations by physicians, and CT scans to identify brain damage, have largely remained unchanged for years. In the fast-paced environment of emergency departments, there is a critical need to triage patients quickly. Now, a novel test that uses whole blood to help assess patients with a suspected mild traumatic brain injury (mTBI), or concussion delivers lab-quality results in 15 minutes. This allows clinicians to conduct assessments at the patient’s bedside, extending its utility to urgent care centers and various other medical settings outside of the hospital emergency room.
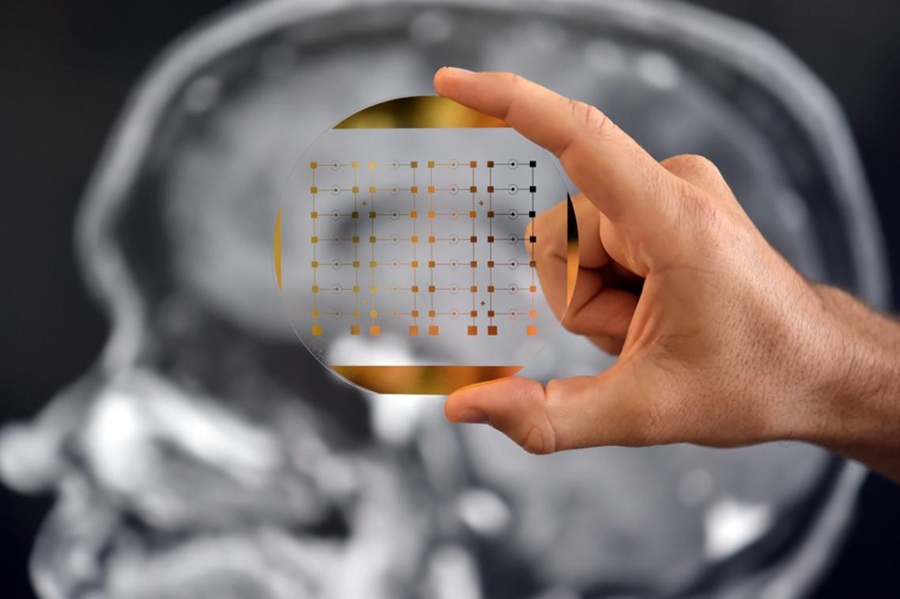
Abbott’s (Abbott Park, IL, USA) i-STAT TBI cartridge has received clearance from the U.S. Food and Drug Administration (FDA) to be used with whole blood, enabling doctors to assess patients with suspected concussions directly at the bedside, and providing lab-quality results in 15 minutes. Unlike previous tests that required plasma or serum, necessitating laboratory processing, the new clearance allows for the implementation of the test in diverse medical environments, including urgent care clinics certified to perform moderate complexity tests. The clearance also marks a significant step towards enabling concussion assessments in non-traditional healthcare settings, such as sports events.

The i-STAT TBI cartridge with the i-STAT Alinity System involves the collection of a small venous blood sample for application to the test cartridge, which is then placed into the portable device. The test quantifies two brain-released biomarkers, ubiquitin C-terminal hydrolase L1 (UCH-L1) and glial fibrillary acidic protein (GFAP), which can signify potential brain injury. These biomarkers offer crucial insights shortly after an injury, aiding clinicians in devising an appropriate care strategy. This whole blood test on the i-STAT Alinity system provides a critical tool for clinicians to assess adults over 18 suspected of suffering from mTBI or concussion.
Results from this test can inform the decision against the need for a CT scan and help in deciding further steps in patient care. The flexibility to conduct the test using a whole blood sample enables its application in medical facilities lacking onsite labs, thus expediting the evaluation process for head injuries. With this clearance, the i-STAT TBI test becomes an important tool in evaluating patients up to 24 hours post-injury, addressing the common delay in seeking medical attention following an injury.
"Clinicians have needed an objective way to assess patients with concussions," said Beth McQuiston, M.D., medical director in Abbott's diagnostics business. "When you look at all the other diseases, or other organs in the body, they all have blood tests to help assess what's happening. Now, we have a whole blood test that can help assess the brain right at the patient's bedside – expanding access to more health providers and therefore patients."