Serum Levels Predict Renal Disease Risk in Diabetes Patients
By LabMedica International staff writers
Posted on 25 Apr 2017
A team of epidemiology researchers has described a prognostic tool that accurately predicts the risk of end stage renal disease (ESRD) in patients with both type I and type II diabetes.Posted on 25 Apr 2017
Design of Phase III trials for diabetic nephropathy currently requires a difficult to obtain group of patients at a high risk of progression defined as within three years of a hard end point (ESRD, 40% loss of estimated glomerular filtration rate, or death). To improve the design of these trials, investigators at Harvard Medical School used natural history data from chronic kidney disease patients with diabetes to develop an improved criterion to identify such patients.
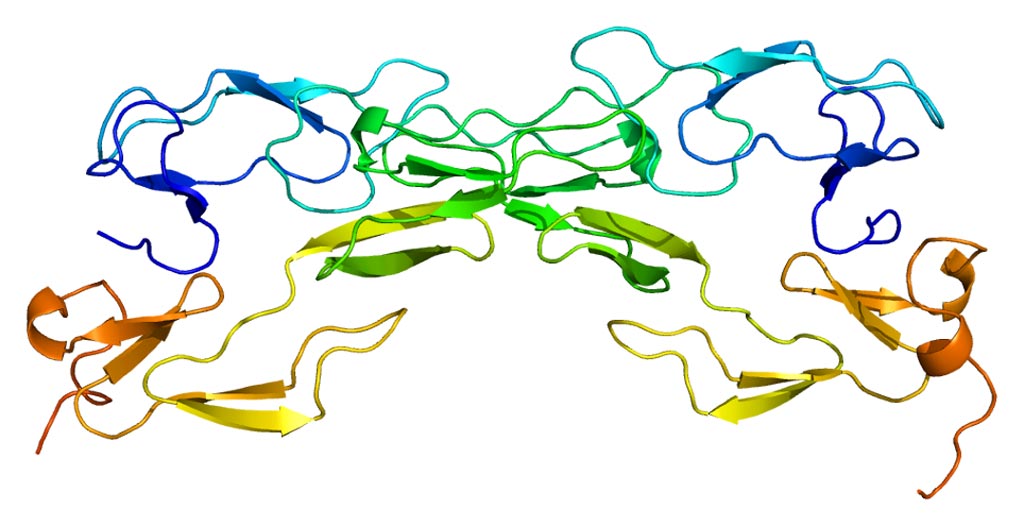
Image: The structure of the tumor necrosis factor receptor 1 protein (Photo courtesy of Wikimedia Commons).
The study group comprised a training cohort of 279 patients with type I diabetes and 134 end points within three years, and a validation cohort of 221 patients with type II diabetes and 88 end points.
Previous trials selected patients using clinical criteria for baseline urinary albumin-to-creatinine ratio and estimated glomerular filtration rate. Application of these criteria to the current cohort data yielded sensitivities (detection of patients at risk) of 70-80% and prognostic values of only 52-63%. The investigators then applied classification and regression trees analysis to select from among all clinical characteristics and markers the optimal prognostic criterion that divided patients with type I diabetes according to risk.
They found that the optimal criterion was a serum tumor necrosis factor receptor 1 (TNFR1) level of more than 4.3 nanograms/milliliter alone or 2.9-4.3 nanograms/milliliter with an albumin-to-creatinine ratio over 1900 milligrams/gram. This criterion produced similar results in both type I and type II diabetic patients. Overall, sensitivity and prognostic value were high (72% and 81%, respectively).
"Overall efficiency and cost effectiveness of clinical trials depends on the diagnostic tools used to enroll study patients," said senior study author Dr. Andrzej S. Krolewski, professor of medicine at Harvard Medical School. "If you recruit people who are not at risk of progressing to ESRD during the clinical trial period, statistical power declines and you cannot prove anything."
"Remarkably, when we used the TNF receptor to analyze risk of ESRD, the risk was almost identical for both type I and type II diabetes. This implies that the etiologies are similar," said Dr. Krolewski. "This is a very important observation because in the medical community, the impression is that the progression to ESRD in type I is somehow different from type II. As a result, many clinical trials do not include patients with type I. Currently, about 80% of patients in these clinical trials provide no useful information. If our criterion is used in the recruitment of patients, you will not need two or three thousand patients for a clinical trial; you will only need 400 patients."
The study was published in the April 7, 2017, online edition of the journal Kidney International.