Rapid Influenza Detection Tests Evaluated for Viral Antigens
By LabMedica International staff writers
Posted on 01 Dec 2016
Reverse transcriptase polymerase chain reaction (RT-PCR) has a high sensitivity and a high specificity for the rapid diagnosis of influenza infection; however, the high cost and necessity of specialized equipment have limited the use of RT-PCR in the clinical setting.Posted on 01 Dec 2016
Immunochromatography-based rapid influenza virus antigen tests, in which the result appears within 15 minutes, have been used for the early diagnosis of influenza in the clinical setting in Japan. Rapid influenza detection tests (RIDTs) equipped with an instrument-based fluorescence reader system has recently been developed, and their performances have been validated.
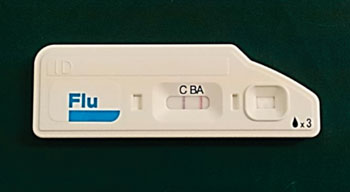
Image: The rapid influenza detection test Quick Navi-Flu (Photo courtesy of Otsuka Pharmaceutical).
Scientists at the Tokyo Medical University (Japan) enrolled a total of 123 participants consisting of 53 men (43.1%) and 70 women (56.9%). The mean age of the patients was 35.1 years (range 20–74 years). The median time to visiting the hospital after illness onset was 26.2 hours (ranging from a few hours to five days). The study was performed from December 2013 to March 2014. Nasopharyngeal swab specimens were collected by performing original swab samplings, which were composed of three swabs.
After collecting the specimens from the participants, the swabs were separated for the tests. One swab was transferred in viral transport medium for virus isolation; the other two swabs were used for the rapid influenza virus antigen detection tests. In this study, the new RIDT GOLD SIGN FLU (Morinaga Milk Industry Co., Ltd, Tokyo, Japan) and Institute of Immunology Co., Ltd, Tokyo, Japan) and the existing immunochromatography-based RIDT Quick Navi-Flu (Otsuka Pharmaceutical Co., Ltd, Tokyo, Japan) were evaluated as compared to viral isolation as the gold standard method.
Among the 123 patients from whom nasopharyngeal swab specimens were collected, 59 tested positive by viral isolation as the gold standard method, 38 with influenza A; 21 with influenza B. For GOLD SIGN FLU, the sensitivities were 73.7% and 81.0%, and the specificities were 97.6% and 98.0% for influenza A and B, respectively. For Quick Navi-Flu, the sensitivities were 86.8% and 85.7%, and the specificities were 98.8% and 100% for influenza A and B, respectively. The time to the appearance of the line on the test strip was less than three minutes for influenza A and less than two minutes for influenza B with both RIDTs in more than 90% of cases.
The authors concluded that GOLD SIGN FLU was useful for diagnosing influenza A, and the result was readily available for influenza B particularly among adult patients. Quick Navi-Flu showed better sensitivities and specificities than GOLD SIGN FLU. The study was published in the November 2016 issue of the International Journal of Infectious Diseases.
Related Links:
Tokyo Medical University
Morinaga Milk Industry
Institute of Immunology
Otsuka Pharmaceutical