Positive Control Developed for Serological Tests for Onchocerciasis
By LabMedica International staff writers
Posted on 03 Feb 2016
Onchocerciasis, or “river blindness,” is a disease caused by the filarial parasite Onchocerca volvulus (Ov) that affects an estimated 37 million in Africa and a few thousand people in the Americas and Yemen. Posted on 03 Feb 2016
Active infection is detected by direct observation of the Ov microfilariae (MF) through skin snip combined with microscopy, but this method is not very sensitive, especially when microfilarial (MF) skin densities are low, which are typical in low-transmission settings.
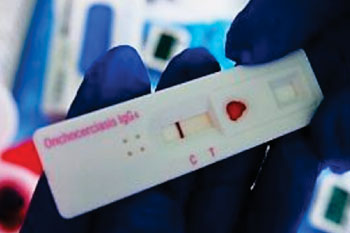
Image: The SD BIOLINE Onchocerciasis IgG4 rapid test preproduction prototype (Photo courtesy of PATH/Dunia Faulx).
Serological assays for human immunoglobulin G4 (IgG) to the O. volvulus antigen Ov16 have been used to confirm elimination of onchocerciasis in much of the Americas and parts of Africa Scientists working at the Diagnostics Global Program (PATH; Seattle, WA; USA) and their international colleagues have standardized a source of positive control antibody (human anti-Ov16 IgG4) that will ensure the quality of surveillance data using these tests.
A recombinant human IgG4 antibody to Ov16 was identified by screening against a synthetic human Fab phage display library and converted into human IgG4. This antibody was developed into different positive control formulations for enzyme-linked immunosorbent assay (ELISA) and rapid diagnostic test (RDT) platforms. Variation in ELISA results and utility as a positive control of the antibody were assessed from multiple laboratories.
When the anti-Ov16 IgG4 antibody was used as a standard dilution in horseradish peroxidase (HRP) and alkaline phosphatase (AP) ELISAs, the detection limits were approximately 1ng/mL by HRP ELISA and 10ng/mL by AP ELISA. Positive control dilutions and spiked dried blood spots (DBS) produced similar ELISA results. Used as a simple plate normalization control, the positive control antibody may improve ELISA data comparison in the context of inter-laboratory variation.
The authors concluded that the recombinant human anti-Ov16 IgG4 antibody-based positive control will benefit inter-laboratory validation of ELISA assays and serve as quality control (QC) reagents for Ov16 RDTs at different points of the supply chain from manufacturer to field use. With the availability of an Ov16 RDT (SD BIOLINE Onchocerciasis IgG4 test; Alere, Standard Diagnostics, Inc.; Yongin, Republic of Korea; www.standardia.com), the ability to monitor the quality of an RDT outside the context of a laboratory throughout the lifecycle of the test in shipping, storage, and use is paramount to having reliable results. The study was published on January 8, 2016, in the journal Public Library of Science Neglected Tropical Diseases.
Related Links:
Diagnostics Global Program
Standard Diagnostics, Inc.










 (3) (1).png)


