Immunochromatographic Test Evaluated to Diagnose Schistosomiasis
By LabMedica International staff writers
Posted on 02 Feb 2016
The Kato-Katz (KK) stool smear is the standard test for the diagnosis of Schistosoma mansoni infection but suffers from low sensitivity when infections intensities are moderate to low and therefore, misdiagnosed individuals remain untreated and contribute to the disease transmission.Posted on 02 Feb 2016
As an alternative, the urine-based diagnosis of schistosomiasis mansoni via the circulating cathodic antigen immuno-chromatographic test (CCA-ICT) has been extensively evaluated in Africa with the conclusion that it may replace the KK test in areas where the prevalence is moderate or high.
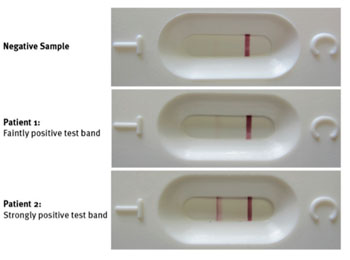
Image: The circulating cathodic antigen immunochromatographic test of two patient’s urine for Schistosoma mansoni (Photo courtesy of Saarland University).
Scientists at the University Vale do Rio Doce (Governador Valadares, Brazil) and their colleagues measured the performance of the CCA-ICT in a sample study population composed of residents from non-endemic and endemic areas for schistosomiasis in two municipalities of Minas Gerais state (Brazil). Volunteers were classified into three infection status groups based on duplicate Kato-Katz thick smears from one stool sample (2KK test): 41 negative individuals from non-endemic areas, 41 negative individuals from endemic areas and 48 infected individuals from endemic areas.
The rapid immuno-chromatographic test used was the commercially available Bilharzia RDT (Rapid Medical Diagnostics; Pretoria, Republic of South Africa), which measures a parasite-derived “circulating cathodic antigen (CCA)” in the urine of patients infected with S. mansoni. Infection status was also determined by the CCA-ICT and infection exposure by antibody enzyme-linked immunosorbent assay (ELISA) to S. mansoni soluble egg antigen (SEA) and soluble (adult) worm antigen preparation (SWAP).
The investigators found that sensitivity and specificity were influenced by whether the trace score visually adjudicated in the CCA-ICT was characterized as positive or negative for S. mansoni infection. When the trace score was interpreted as a positive rather than as a negative result, the specificity decreased from 97.6% to 78.0% whereas sensitivity increased from 68.7% to 85.4%. A significantly positive correlation between the CCA-ICT scores and egg counts was identified. Considering 2KK as reference test, the discriminating power of the CCA-ICT was greater than the SEA-ELISA and SWAP-ELISA.
The authors concluded that the performance of the CCA-ICT in the Brazilian communities endemic for schistosomiasis mansoni support those from Africa in areas with greater infection prevalence and intensities, the CCA-ICT may be useful as a tool to indicate community-based preventative chemotherapy without individual diagnosis. However, because of the Brazilian Ministry of Health’s recommendation for individual diagnosis in areas where prevalence is less than 15%, in those areas in which infection intensities are likely to be lowest, the CCA-ICT lacks the sensitivity to be used as standalone diagnostic tool. The study was published on January 11, 2016, in the journal Public Library of Science Neglected Tropical Diseases.
Related Links:
University Vale do Rio Doce
Rapid Medical Diagnostics












