Neglected Malaria Subspecies Characterized by Novel Molecular Assay
By LabMedica International staff writers
Posted on 27 Jan 2015
Plasmodium ovale subspecies are similar based on morphology and geographical distribution, but allelic differences indicate that P. ovale curtisi and P. ovale wallikeri are genetically divergent.Posted on 27 Jan 2015
Potential clinical and latency duration differences between P. ovale curtisi and P. ovale wallikeri demonstrate the need for investigation into the contribution of this neglected malaria parasite to the global malaria burden.
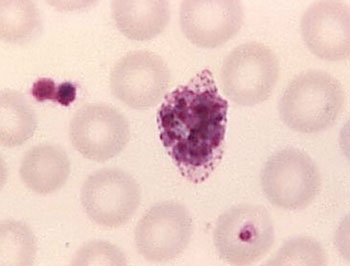
Image: A mature Plasmodium ovale trophozoite in a thin blood film (Photo courtesy of the CDC – US Centers for Disease control and Prevention).
Scientists at the Uniformed Services University (Bethesda, MD, USA) working with colleagues in Kenya, collected whole blood samples from clinically healthy asymptomatic adult individuals in Nyanza Province, Kenya. The samples were screened with the Parascreen Pan/Pf malaria Rapid Diagnostic Test (Zephyr Biomedicals, Verna, Goa, India) for the presence/absence of malaria parasites from March through September of 2008. Thin and thick smears were examined subsequently by up to five expert microscopists for malaria species designation and estimation of quantitative Parasitemia.
The 22 samples identified as positive for P. ovale via microscopy, in which all were mixed infections with other malaria species, were targeted for DNA extraction and polymerase chain reaction (PCR) based analysis. The team used multilocus genotyping to discriminate P. ovale curtisi and P. ovale wallikeri and all conventional PCRs were performed on a DNA Engine PTC-200 Thermal Cycler (MJ Research; Waltham, MA, USA). Real-time PCR were conducted on the on the ABI 7500 fast real-time PCR (qPCR) platform (Life Technologies; Carlsbad, CA, USA).
Alignments of tryptophan-rich antigen (tra) gene sequences revealed nine samples (40.9%) positive for P. ovale curtisi type 1, two samples (9.1%) positive for P. ovale curtisi type 2, six samples (27.3%) positive for P. ovale wallikeri type 1, and three samples (13.6%) positive for P. ovale wallikeri type 2. Specificity studies showed the ability of the reticulocyte binding protein 2 (rbp2) qPCR assays to detect low-levels of P. ovale in the presence of additional malaria parasite species, including P. falciparum, P. vivax, and P. malariae. The team identified P. ovale curtisi and P. ovale wallikeri in Western Kenya by DNA sequencing of the tryptophan-rich antigen gene, the small subunit ribosomal RNA gene, and the rbp2 gene.
The authors concluded that their novel P. ovale rbp2 qPCR assay detects P. ovale curtisi and P. ovale wallikeri simultaneously and can be utilized to characterize the prevalence, distribution, and burden of P. ovale in malaria endemic regions. The use of multilocus genotyping also provided the first description of the prevalence of P. ovale curtisi and P. ovale wallikeri in Western Kenya, a region holoendemic for malaria transmission. The study was published on January 15, 2015, in the journal Public Library of Science Neglected Tropical Diseases.
Related Links:
Uniformed Services University
Zephyr Biomedicals
MJ Research













